The problem of bias is well documented in the biosciences. Even since the Health Revitalization Act of 1993, which laid out guidelines intended to ensure more equitable representation of women and minorities in federally funded scientific research, the problem persists. A 2010 study published in The Journal of Women’s Health found that, among 46 clinical studies enrolling both sexes, women comprised on average 37% of the participants, and among 69 studies, 87% did not conduct analyses by race or ethnicity, and 18% did not report differences in the racial makeup of the study sample at all. Examples of this sort abound and, setting aside the pernicious sociohistorical and nuanced biologic reasons for this phenomenon, the resulting reality is that medicine, as applied to women and minorities, is less evidence based because most research is extrapolated from a homogeneous population—white men.
But even as we attempt to resolve these problems—ensuring that guidelines are in place and that they are followed when conducting new research—there is another, more subtle way that these biases creep into the biomedical literature. Even if the study itself was conducted using a diverse population of participants, sometimes the reporting elides this fact. As a manuscript editor I have encountered this problem more often than one would expect, and the culprit is usually the table.
In this table, as in many tables that I have encountered, “white” and “male” are the default. Women’s bodies and the bodies of racial and ethnic minorities are implied by the number of white male bodies present.
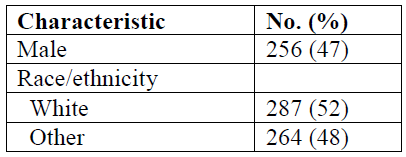
A good rule when presenting data in tables is to make sure that when you are reporting the sex of participants, if you choose to report only 1 sex, choose the sex that constitutes the majority of the sample. When reporting on racial and ethnic differences, be as specific as possible (even if these comprise a small percentage of participants). Who are the “others?”
The current edition of the AMA Manual of Style does not explicitly lay out these precautions, but in chapter 4, section 1, you will notice that every example shown for presenting data in tables follows these guidelines.
This is not merely a problem of “political correctness” or social equity—it is a question of accurate reporting and just plain good science.—Gabriel Dietz

